Study summary
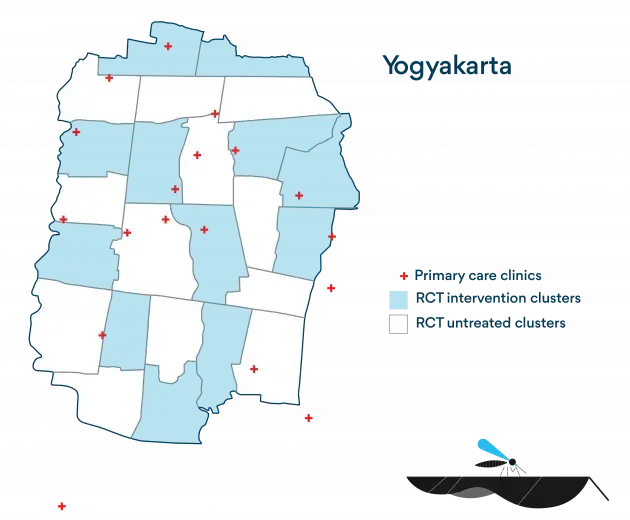
In order to measure the efficacy of the Wolbachia method, WMP, in collaboration with partners the Tahija Foundation and Gadjah Mada University, conducted a Cluster Randomised Controlled Trial within a 26km2 area of Yogyakarta City, Indonesia.
The aim of the study was to determine whether deployment of Wolbachia Aedes aegypti mosquitoes leads to a reduction in dengue incidence in Wolbachia-treated versus untreated areas. The study site was subdivided into 24 clusters, among which 12 were randomly selected to receive Wolbachia mosquito releases alongside routine dengue control measures alongside routine dengue control measures. The remaining 12 continued to receive routine dengue control measures.
Following the successful establishment of Wolbachia, consenting patients presenting with fever were enrolled at a network of primary care clinics across the study area over a period of 27 months, and were tested for dengue.
In June 2021, the New England Journal of Medicine published the peer reviewed results of the trial which show that Wolbachia deployments reduced dengue incidence by 77% and dengue hospitalisations by 86%.
*(95% confidence interval: 65% to 85%)
Read the factsheet:

Summary of findings
Over the course of the trial, 318 dengue cases were detected in the untreated areas of the trial site and only 67 in the Wobachia treated areas, which translates to a 77% reduction in dengue incidence in the treated areas of the city.
Efficacy against hospitalised dengue was even higher at 86%, with only 13 hospitalisations for dengue in the Wolbachia-treated area compared to 102 in the untreated area.
All four dengue virus serotypes were detected among trial participants and efficacy was similar across them all.



FAQs
(Download all the FAQs here)
- 1Why Yogyakarta?
Yogyakarta has regularly ranked among the top ten provinces in Indonesia for annual dengue incidence, over the past three decades. The World Mosquito Program and the University of Gadjah Mada, Yogyakarta, have since 2011 been working with local government, health authorities and communities to undertake pilot deployments of Wolbachia in Yogyakarta province. These pilot studies have demonstrated successful and durable Wolbachia establishment, with strong community support. These factors, together with a strong health system and human resource capacity for high quality clinical research, made it well-suited as the site for the first efficacy trial of WMP’s Wolbachia method.
- 2What is the key finding from the study?
The efficacy of Wolbachia (wMel strain) against virologically-confirmed dengue among persons aged 3-45 years was 77%. This means that dengue incidence was 77% lower among persons who lived in a Wolbachia-treated area compared to those who lived in an untreated area during the 27 months of the trial. Importantly, the incidence of dengue requiring hospitalisation was 86% lower in the Wolbachia-treated area than the untreated area.
All four dengue virus serotypes were detected among trial participants, and protective efficacy was similar across serotypes. Efficacy was highest for serotype 2 (84%) and lowest for serotype 1 (71%).
- 3What is a cluster randomised controlled trial?
A cluster randomised controlled trial is considered a gold-standard method for evaluating the efficacy of health interventions delivered at the community level. It involves the random allocation of an intervention to a subset (e.g. 50%) of predefined spatial units, and the comparison of disease outcomes amongst individuals living in areas with versus without the intervention. The random allocation of the intervention results in two study arms, one treated, one untreated, that are comparable at baseline in all factors except for the intervention under study. This allows for an unbiased estimate of the effect of the intervention on the disease of interest.
- 4What is different about a test-negative cluster randomised controlled trial?
A test-negative design differs from a traditional cluster randomised trial in the way the disease outcomes are detected. Instead of recruiting a cohort of participants from treated and untreated communities and following them up over time to detect disease outcomes, a test-negative design recruits participants from among patients who present at healthcare facilities with a particular clinical syndrome, in our case a fever, and determines using a laboratory test who is positive or negative for the disease of interest (dengue). The efficacy of the intervention is then determined by comparing the likelihood of having received the intervention between persons who tested positive and those who tested negative. It can be more efficient, cheaper and logistically simpler to implement than a traditional cluster randomised controlled trial as it does not require prospective follow-up of thousands of participants over many years to attain a sufficient number of dengue cases for robust statistical analysis. Also, because all study participants are recruited from within the population of patients who present to a healthcare facility, it reduces the possibility of bias that may result from any differences in care-seeking behaviour between persons who did and did not receive the intervention.
- 5How were the treatment areas for the study selected?
The study area was subdivided into 24 contiguous clusters, each with an area of approximately 1 km2 and an average population of 13,000 people. Among these 24 clusters, 12 were randomly allocated to receive Wolbachia deployments and 12 to remain untreated. This random assignment of the intervention was ‘constrained’ to ensure balance between Wolbachia-treated and untreated areas for some key factors including historical dengue incidence, incidence of non-dengue febrile illnesses, population demographics (proportion aged <15 years, socioeconomic status), population size, and area size. The final random allocation was drawn in a public randomisation event involving community leaders, to maximise transparency and acceptability of the process.
- 6Where do the Wolbachia mosquitoes come from?
The Wolbachia mosquitoes are from a mosquito colony that has been maintained in Yogyakarta since 2013. Wolbachia was first introduced into mosquitoes from Yogyakarta by mating wild-type male Ae. aegypti mosquitoes from Yogyakarta with female Wolbachia-infected mosquitoes from Australia for 5 generations. This is called backcrossing and it serves to replace the Australian genetic background with the local Indonesian genetic background. Frequent outcrossing of the colony since 2013 with local wild-type mosquitoes ensures the genetic background of the mosquitoes is always well-matched to the mosquitoes flying around in Yogyakarta.
- 7When and how were the Wolbachia mosquitoes deployed in Yogyakarta?
Wolbachia mosquitoes were released as eggs between March and December 2017. Households hosted these mosquito release containers, to which Wolbachia mosquito eggs, water and fish food were added once every two weeks, for 4-6 months. Adult Wolbachia mosquitoes emerged from the containers, then bred with wild-type Ae. aegypti mosquitoes until almost all Ae. aegypti in the intervention areas carried Wolbachia.
- 8How do you prevent the Wolbachia mosquitoes from flying into untreated areas?
Natural borders (roads, rivers, non-residential areas) were used to define cluster boundaries as much as possible, to limit the spatial spread of Wolbachia into untreated areas. Some contamination of untreated clusters did occur, and this is accounted for in a secondary (per-protocol) analysis that will be published as part of the detailed trial results in a peer-reviewed journal.
- 9Did the Wolbachia contamination in untreated areas affect the results of the trial?
Some contamination of Wolbachia into ‘untreated’ clusters did occur, which means that some people classified as living in an untreated area may have had a partial protective effect from Wolbachia. This would be expected to dilute the true intervention effect, which means our result may be an underestimate of Wolbachia’s true effect on dengue incidence.
- 10How can you know whether participants got their dengue infection in a Wolbachia-treated or untreated area?
The primary (‘intention-to-treat’) analysis that produced the 77% efficacy result classifies participants as simply Wolbachia-exposed or not, based on whether their residence was in an area that received Wolbachia deployments or not. We collected information from participants at enrolment about the locations where they had spent time during the 10 days prior to illness onset, from which we can determine the proportion of their time spent in Wolbachia-treated and untreated areas.
Efficacy was equivalent in a secondary (‘per protocol’) analysis accounting for time spent outside the cluster of residence, suggesting that the home is a primary location for dengue risk.
- 11Did the trial require that other routine vector control activities stop in intervention areas and/or untreated areas?
No, Wolbachia deployments were in addition to routine vector control activities. No changes were introduced to existing activities in either the intervention or untreated areas.
- 12Besides Wolbachia were there differences in mosquito-control activities between the treated and untreated areas?
Routine vector control measures are focused on community-based activities to reduce mosquito breeding sites, application of larvicide and focal insecticide fogging in areas around notified cases. These activities are managed at the village level and their implementation varies somewhat between communities, but the community engagement messaging both before and during the trial emphasised that usual vector control and bite prevention practices should be continued throughout both intervention and untreated areas.
- 13Were the communities blinded to whether they were in a treated or untreated cluster? If not, how do you know that this didn’t affect the results of the trial?
The nature of the Wolbachia intervention means that blinding was considered infeasible, as it would have doubled the resources and time required to conduct field releases of mosquitoes; for example, with inactive eggs as the placebo. The risks to study validity from a non-blinded deployment (for example, if a belief that Wolbachia is protective against dengue cases leads residents of treated areas to be less likely to seek care for a febrile illness) were also seen to be minimised by the fact that dengue cases and test-negative controls are drawn from the same patient population, who are clinically indistinguishable at the time of presentation and enrolment at clinics. The test-negative cluster randomised controlled trial design is tolerant to the possibility that healthcare seeking behaviour is modified by a person’s knowledge of their Wolbachia status, as long as this modified behaviour applies equally to test-positive dengue cases and test-negative controls.
- 14Did you have consent from all residents of the treated areas to release Wolbachia mosquitoes in their community?
Extensive effort was invested in local community engagement leading up to this trial, extending from community leaders and key stakeholders to the media and the general public, with an aim of informing the community about the planned Wolbachia releases and addressing any concerns. Approval for releases was given by community leaders after extensive community consultation, with individual residents’ consent obtained for hosting a mosquito release container at their property.
- 15Will the intervention eventually eliminate dengue from Yogyakarta?
On the basis of the positive trial results, the District Health Office of Yogyakarta together with the World Mosquito Program in Indonesia completed Wolbachia deployments throughout the untreated areas of Yogyakarta city, in January 2021. Releases are underway in the neighbouring Sleman and Bantul districts of Yogyakarta province in 2021-22. Once Wolbachia is durably established throughout Yogyakarta city, it is conceivable that this could lead to local elimination of dengue transmission for years to come, although enhanced case finding and diagnosis would be needed to track progress towards and achievement of any elimination goal.
- 16Was the efficacy of Wolbachia equivalent for all four dengue virus serotypes?
All four dengue virus serotypes were detected among trial participants, and protective efficacy was similar across serotypes. Efficacy was highest for serotype 2 (84%) and lowest for serotype 1 (71%).
- 17What do these results mean for other dengue endemic settings?
These results are consistent with published findings from non-randomised deployments in northern Queensland, Indonesia, and Brazil. This gives us confidence that the reduction in dengue incidence reported here is likely to be replicated across different epidemiological settings. It also supports evaluation of the impact of large-scale Wolbachia deployments using routinely available disease surveillance data, since it is neither feasible nor necessary to conduct a formal efficacy trial in each of the many settings in which Wolbachia deployments are likely to be of benefit.
It is possible that differences in the predominance of circulating DENV serotypes in different locations may influence the generalisability of these results to other settings, but in general we expect that in other locations where Wolbachia is established at a high level, we will see similarly significant reductions in local arboviral disease incidence. Indeed, where Wolbachia deployments are being conducted across a larger contiguous area, we expect the reduction in disease burden to be even greater because the vast majority of people’s movements (and mosquito exposure) will be within the Wolbachia-treated area.
The main caveat in replicating these results elsewhere is that differences in ecology, climate, altitude and the complexity of the urban environment are likely to affect the trajectory of Wolbachia establishment, and consequently the timing of the impact on disease.
- 18What does the 95% confidence interval tell us?
Because the true intervention effect is unknown, the 95% confidence interval provides a range of possible values. In this study, our best estimate of the intervention effect is the reported point estimate of 77%. If this study could be repeated many times, we would likely see slight variation in each repeated study’s estimate of the true intervention effect due to sampling variability. The 95% confidence interval is constructed such that if this study were repeated many times, 95% of intervals estimated this way would contain the true intervention effect.
- 19If my city or my country wants to deploy Wolbachia, how can we get involved?
If you would like to deploy Wolbachia in your city, please email us at contact@worldmosquito.org with information about you, your organization and how you would like to get involved.